CIKLab is an online solution allowing you to organise your control plans and easily track your quality...
Many food safety analyses are outsourced to independent laboratories, often accredited to the ISO/IEC 17025 standard. What should be included in a test report, and what should be verified?
Summary: 
1) Understanding ISO 17025 accreditation, the role of COFRAC, and recognition agreements
2) What should be included in a test report?
3) How to read an analysis report ?
Many food safety analyses are outsourced to independent laboratories, often accredited to the ISO/IEC 17025 standard by COFRAC. The analysis reports, or "test reports," issued by these laboratories naturally include the results of self-checks, and as an operating industrial entity or requesting client, you must ensure that you verify a number of elements.
Working with accredited laboratories or conducting analyses under accreditation is not a requirement for self-checks, although it is advisable. A non-accredited laboratory must, at the very least, perform interlaboratory tests to demonstrate its competence in the relevant tests.
Understanding ISO 17025 accreditation, the role of COFRAC, and recognition agreements
Accreditation is the formal recognition of a laboratory's technical competence to perform specific tests in accordance with the international standard ISO/IEC 17025. To be very specific: COFRAC accreditation attests to the laboratory's competence only for the tests covered by the accreditation. These tests or analyses are listed in the technical annex to the accreditation certificate. The accreditation certificates and technical annexes for each accredited laboratory are available on the COFRAC website.
Compliance with ISO/IEC 17025 requires laboratories to demonstrate their competence, impartiality, and integrity.
Working with an accredited laboratory ensures that the laboratory provides results according to the highest "quality" standards.
In France, COFRAC accredits laboratories.
COFRAC, which stands for the French Accreditation Committee (Comité Français d'Accréditation), is the national body responsible for accrediting testing laboratories, calibration laboratories, certification bodies, and inspection organizations in France. Its primary role is to ensure the technical competence and impartiality of these laboratories and organizations.
Each accredited laboratory receives an accreditation certificate with a unique number, and the technical annex to the certificate lists the analytical tests (method and food matrix involved) for which the laboratory is accredited. This is known as the scope of accreditation. The accreditation status of a laboratory can be verified on the COFRAC website. Laboratories undergo regular audits in accordance with ISO 17025, ensuring their ongoing competence.
Internationally, how does accreditation work?
Laboratories operating outside of France are not accredited by COFRAC but are accredited against the same ISO/IEC 17025 standard by other national accreditation bodies, such as UKAS in the United Kingdom, BELAC in Belgium, etc.
The accreditation provided by these national bodies is equivalent if they are signatories of recognition agreements.
- In Europe, EA (European co-operation for Accreditation) is the organization that brings together accreditation bodies that are signatories to recognition agreements (MLA for Mutual Recognition Agreement).
- Internationally, ILAC (International Laboratory Accreditation Cooperation) also has a mutual recognition arrangement (MRA) that ensures that accreditations issued by an ILAC member are recognized by all other members.
ISO/IEC 17025 accreditation and recognition agreements enhance confidence in international trade. Businesses and governments can rely on test and analysis results because they are based on internationally recognized and comparable standards regardless of the country of origin. This confidence facilitates international trade and continually improves the safety and quality of traded products
💡 Test reports from two laboratories accredited by accreditation bodies signatory to recognition agreements are equivalent!

What should be included in a test report?
A test report or analysis report is a crucial document in the field of food safety. It presents the results of analyses conducted by independent accredited (or non-accredited) laboratories on samples submitted for analysis. This report must adhere to very strict rules outlined in the ISO/IEC 17025 standard. Many laboratories tend to use the same "format" for reports, aligning with this standard, even if they are not accredited.
What are the mandatory elements on a test report?
The ISO/IEC 17025 standard clearly specifies the elements that must be included in a test report, particularly in Chapter 7.8.2, "Common Requirements for Reports."
A test report typically includes the following mandatory elements:
-
- Title: For example, "Test Report," "Analysis Report," or "Certificate of Analysis."
- Unique Report Number: A distinct report number for identification purposes.
- Client's Contact Information: Contact details of the client or the requester of the analysis.
- Laboratory Information: Name and address of the laboratory, including its accreditation number.
- Location of Activities: This can be the laboratory itself or, in some cases, on-site at your facility if in-situ measurements are taken.
- Unique Identification: To ensure that all elements are considered part of a single report, such as "this report comprises 3 pages."
- Method Identification:
- Appropriateness: The method should be suitable, and if no specific method is specified, the laboratory will choose the most appropriate one.
- Up-to-Date: The laboratory must ensure the use of the latest version of a standard, for example.
- Validated: With evaluation of associated uncertainty.
- Clear Indication of Deviations: Any deviations from the methods must be clearly indicated or negotiated and agreed upon in advance with the client.
- Description and Unambiguous Identification: Clear and unambiguous identification of the object, which is your sample.
- Sample-Related Dates: The date of receipt of each sample submitted for testing, the sampling date if relevant for validity, and the application of results.
- Analysis Date: The date when the analysis was conducted.
- Report Issuance Date: The date when the analysis report is issued.
- Reference to Sampling Plan and Method: Reference to the sampling plan and method used by the laboratory if it performed the sampling.
- Statement of Limited Scope: A declaration that the results pertain only to the samples submitted for analysis, and it's the responsibility of the requester to validate the sample's representativeness.
- Results:
- Measurement Units.
- Measurement Uncertainty, expressed in the same units as the result (measurand) or as a relative percentage (U is displayed if required by the client, for validity, or to assess compliance with a specification or requirement).
- Analysis Conditions Information: Any information regarding analysis conditions, such as temperature, if applicable.
- Opinions or Interpretations: To be provided by authorized individuals only.
- Authorizing Person Identification: Identification of the person or persons authorizing the report.
- Clear Identification of External Provider Results: For results from external providers in case the laboratory subcontracts certain analyses.
- Restriction on Reproduction or Communication: A mention that the report can only be reproduced or communicated in its entirety with prior approval from the laboratory to prevent results from being shared out of context, for example
What should be checked before sending a sample for analysis?
Before sending a sample for analysis, you should pay particular attention to several elements when outsourcing food analyses:
Sample Representativeness: Ensure that the sample you send for analysis is representative. Keep in mind that a laboratory will always provide an analysis result for a sample and not for an entire batch, product, brand, etc.
Required Sample Quantity: Consider the minimum sample quantity needed for analysis. While there is a technical minimum required, sometimes a larger quantity may be necessary, especially for analyses conducted under accreditation or official controls (e.g., mycotoxins, pesticides).
Accurate Sample Identification: Provide the most precise identification of your sample.
Clear Analysis Request: Clearly specify the desired analysis, which is inseparable from a method (e.g., NF EN ISO standard), a technique, or a reference with your laboratory.
Specified Content or Limit: If applicable, provide a specification for the content or an expected limit, which should be associated with the unit in which the result will be reported. This allows you to know what you will compare your analysis result to in order to assess its conformity.
💡 Use a clear and detailed purchase order to address all these points. An internal order number should enable you to track the entire traceability of your request. It should also contain all contractual elements such as price, delivery time, report delivery address, contact person, etc
How to read an analysis report ?
Not all laboratories are accredited, and not all accredited laboratories issue results under accreditation. In any case, the format of analysis reports tends to standardize, even for non-accredited laboratories.
As a "requesting client," here is what you should check at a minimum:
Identification :
If the laboratory is accredited, its accreditation number should generally appear along with the COFRAC logo. Your sample should also have an identification number, as should the report. The test report should include a detailed description of the analyzed sample. This description should include the information you provided in advance, such as the sample's origin, sampling date, lot number, product article code, and any other details that can help identify your sample. Certain conditions may also be specified to provide additional information for the result (e.g., temperature, packaging unit, etc.).
If results are issued under accreditation, they must clearly be distinguished from other results (with an asterisk, an "A," a "*", a checkmark, etc.) and include a statement like "tests covered by accreditation.
Résults :
The report must clearly present the results of the analyses conducted on the sample without ambiguity. These results are typically expressed in specific units of measurement, and it's important to verify that the units used are identical to those specified in current regulations or professional practices. This ensures result comparability, interpretation consistency, and reduces the risk of errors.
💡 For example, if you're using units like ppb (parts per billion) for Heavy Metals analysis (which occurs in some countries) and the analysis result is related to a regulation like EU Regulation No. 915/2023, there's a high chance the laboratory will express the result in mg/kg, which could pose a conversion issue for assessing compliance.
The names of the tested analytes should also be unambiguous. If a chemical element is being tested, clear identification with the CAS number is valuable, just as the scientific name of certain pathogenic microorganisms.
⚠️ Be cautious when working with Anglo-Saxon laboratories regarding the presentation of results with separators:
For example, "Total Plate Count: 1,000 CFU/g" might appear on an Anglo-Saxon report like this and 1 000 UFC/g in French
Uncertainties
The test report may include (although it's not a specific requirement) measurement uncertainties for each result. Uncertainties are often expressed in the result's unit in the format "+/-" with expanded confidence intervals (K=2 at 95%). They reflect the reliability of the measurements, taking potential errors into account.
Uncertainties can have a significant impact when assessing compliance with a regulatory limit. As a requester, you must absolutely take them into account, especially in cases of overlap, for example:
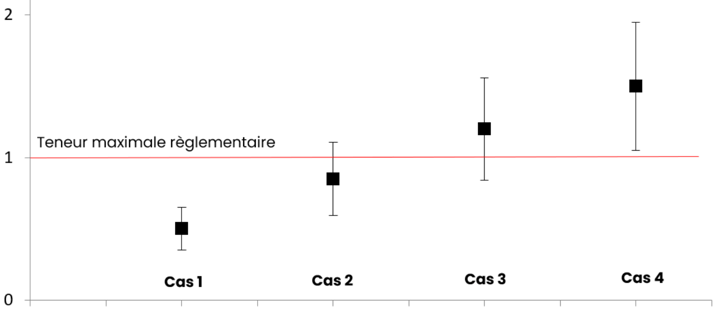
- Case #1 = Value conforms to the regulatory limit with uncertainty: The result, when accounting for uncertainty (+/-), is still within the regulatory limit.
- Case #4 = Value does not conform to the regulatory limit with uncertainty: The result, even when considering the uncertainty (+/-), exceeds the regulatory limit.
- Case #2 = Value conforms with a "risk" of non-conformity due to higher uncertainty: The result is within the regulatory limit, but the relatively high uncertainty increases the risk of non-compliance.
- Case #3 = Value does not conform with a "risk" of conformity due to lower uncertainty: The result falls outside the regulatory limit, but the relatively low uncertainty decreases the risk of non-compliance.
In the case of "limit values" where the uncertainty overlaps with the limit:
- Uncertainty should be subtracted from the value before determining regulatory compliance in certain cases, such as for analytes that do not cause acute toxicity (Case #3 becomes compliant).
- However, uncertainty should not be subtracted in the case of analytes that pose a risk of acute toxicity (e.g., marine biotoxins, alkaloids) (Case #3 remains non-compliant).
⚠️ Reference to French technical note DGAL/MUS/2023-11, among others, provides detailed information on this topic.
Abbreviations commonly used in analysis reports
In many analysis reports, especially in analytical chemistry, abbreviations are commonly used. It's important to detail and translate these abbreviations to avoid any misinterpretation. For example:
- ppm = mg/kg (Parts per million = Milligrams per kilogram)
- LoQ / LQ = Limit of Quantification
- LoD / LD = Limit of Detection
- HAP = Polycyclic Aromatic Hydrocarbons
- ST = Subcontracted
- Ne = Estimated Number
- ND = Not Detected
- U = Uncertainty
- %RSD: Percent Relative Standard Deviation (a measure of precision).
- CAS: Chemical Abstracts Service (a registry for unique chemical compounds).
Analytical methods
The test report should also include information about the analytical method used. This is crucial for verifying the validity of the results, giving them a certain level of credibility, and allowing for result reproduction in another laboratory for result comparison.
Likewise, accredited laboratories must follow standardized methods such as ISO, AOAC, Eur.Ph, etc., to ensure the reliability and reproducibility of results since these methods are already well-established. In cases where non-standard or in-house methods are used, they should be validated.
💡 Be cautious about method selection, especially regarding what is to be measured. Some methods may have low specificity (colorimetric and spectrophotometric methods), while others are much more precise and specific (methods based on LC-MS/MS).
Lab comments
A test report can also include comments and recommendations, especially if non-conformities have been detected. This information can be essential for companies to take the necessary actions to ensure the safety and quality of their products. Only authorized individuals designated for this purpose can provide recommendations and comments. However, it's important to note that the laboratory will base its recommendations and comments solely on the results related to the sample to provide an opinion.
💡 In case of doubt or if you have questions, don't hesitate to contact the laboratory to discuss the results, regulations, and other relevant matters. Your subcontractors are usually open to communication, and you can learn a lot through these exchanges !
©2024 CIKLab.com All rights reserved.






